Simulation of Methanol and Urea Production from Catalytic Conversion of Steel Mill Gases
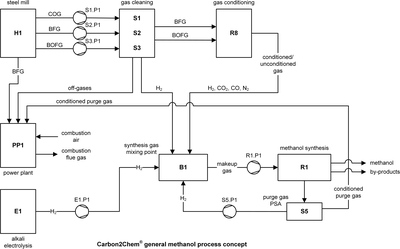
The time-dependent operation of methanol, ammonia, and urea production units embedded in a steel mill technical environment is analyzed with dynamic simulation models. From different process concepts and gas availability scenarios a set of simulation cases is defined with blast furnace gas (BFG) as carbon and coke oven gas (COG) as hydrogen source. The simulation scenarios for the distributed cross-industrial network are based on time-dependent gas availability data from the Duisburg site of thyssenkrupp AG. In the Carbon2Chem® simulation plan, gas availability data and process concepts are combined to specific simulation scenarios. The present work considers different BFG usage flow rates, different BFG gas conditioning (cleaning only, water-gas shifting), classical carbon capture and utilization (CCU) from power plant exhaust gas and different hydrogen availability concepts. The CO-rich basic oxygen furnace gas (BOFG) is not considered in any case, as this gas is a relevant part of the steel mill processing. The chemical product is methanol (synthesized from CO, CO2 and H2) or urea (synthesized from NH3 and CO2). The ammonia synthesis run with H2 and N2 from BFG after a water-gas shift process. In Fig. 1 the standard concept for the production of methanol from BFG and COG is shown. The simulation cases were set up and parametrized in COMSOL Multiphysics®; important settings are given in the presentation. The COMSOL Multiphysics® model is build up from up to 50 single models of technical/chemical units organized as separate COMSOL® components. These single units are connected together into a large model file to build up the block flow diagram of the process. So, COMSOL Multiphysics® is used as a very powerful flowsheet simulator. In a first steady-state run the process flow-sheet data are calculated with the yearly average values of the steel mill gas flow rates. Most relevant result flows like makeup gas, synthesis product, hydrogen production, or power plant operation are checked and validated against previous results and feasible data (computation time in the range of minutes). Next, a time-dependent simulation run over a whole production year is started on a Fujitsu® simulation cluster running COMSOL Multiphysics® (computation time 1–3 days). The dynamic simulation of methanol and ammonia/urea production from steel mill gases shows the feasibility of this CO2 reduction method. Main limiting factors are the availability of raw gases, the availability of external hydrogen for synthesis gas conditioning and certain thermodynamic synthesis conditions like pressure and inert gas content. We confirm and highlight the importance of the green hydrogen for the large scale process concepts as mentioned in other publications. From the results, global data such as carbon footprint or energy demands and details about process unit operation are obtained and processed.
Download
- cite.202000068.pdf - 4.54MB
- Presentation_Schlueter_COMSOL_Conference_Europe_2020.pptx - 13.85MB
